MIT chemical engineers have pioneered a method to transform carbon dioxide into carbon monoxide, a key intermediary for synthesizing valuable chemicals like ethanol and other fuels. This innovation, if expanded to industrial scales, holds the potential to significantly mitigate carbon emissions from power plants and other sources by converting CO2, a prominent greenhouse gas, into commercially viable products. Ariel Furst, the lead researcher and an assistant professor of Chemical Engineering at MIT, underscores the importance of this development as a stride towards decarbonization, highlighting the conversion of CO2 into beneficial chemical products.
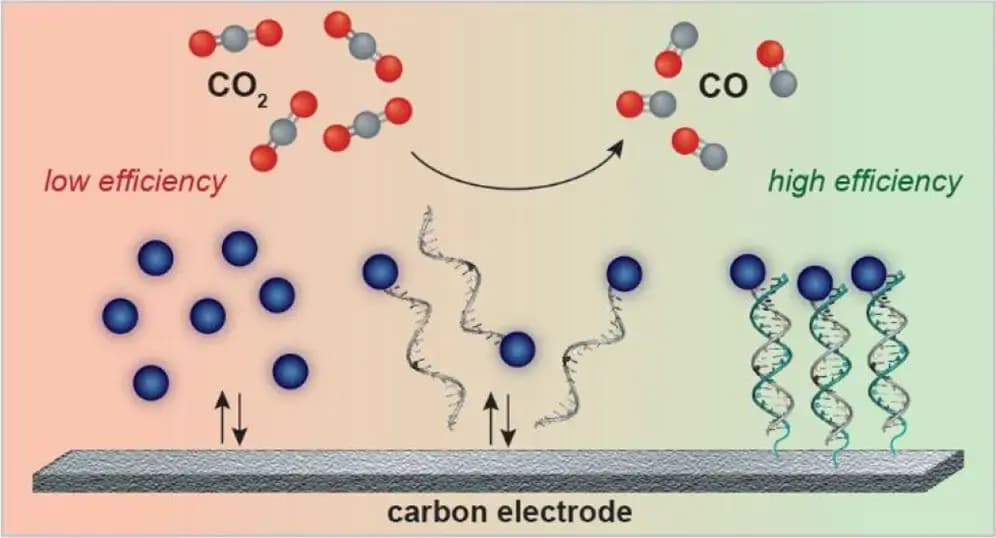
The novel technique employs electrical energy to facilitate the conversion, leveraging a catalyst anchored to the electrode through DNA strands. This DNA-based attachment creates a close-knit environment for the reaction components, enhancing the efficiency of the conversion process compared to traditional methods where components are dispersed in a solution. Furst has founded Helix Carbon to advance this technology, with the research findings published in the Journal of the American Chemical Society Au, co-authored by former MIT postdoc Gang Fan and others.
The process centers on the electrochemical conversion of carbon dioxide to carbon monoxide, a step traditionally hindered by high energy demands. The use of electrocatalysts, particularly porphyrins containing metals like iron or cobalt, has been explored to accelerate this reaction and lower energy requirements. However, the efficiency of these reactions has been limited due to the infrequent interactions between CO2 and the catalysts at the electrode surface.
Furst’s team has innovated by attaching the catalysts directly to the electrode surface using DNA, which acts like a highly selective and controllable Velcro, ensuring frequent and efficient reactions. This system allows for the carbon dioxide dissolved in water within an electrochemical device to be converted into carbon monoxide more effectively, with the added benefit of generating a small amount of hydrogen gas.
The efficiency of this method has been significantly enhanced, achieving a Faradaic efficiency of 100%, meaning all the electrical energy is effectively utilized in the reaction, a stark improvement from the 40% efficiency without DNA tethering. This advancement not only promises greater affordability due to the use of inexpensive carbon electrodes and catalysts but also opens up possibilities for producing a variety of chemical products by changing the catalysts. Furst’s company, Helix Carbon, is poised to further develop this technology for commercial application, backed by funding from several prestigious institutions.
The header image is courtesy of the researchers and JACS Au.
The original media release can be accessed here.
The original research article can be accessed here.









Responses (0 )