Neutrons, known for their lack of electric charge, are not subject to electromagnetic forces like their counterparts, protons and electrons. This unique characteristic has led MIT researchers to a groundbreaking discovery: neutrons can bind to quantum dots solely through the strong force, which typically only operates at the atomic nucleus level. This finding, recently reported in ACS Nano, presents potential new avenues for quantum-level material probing and quantum information processing.
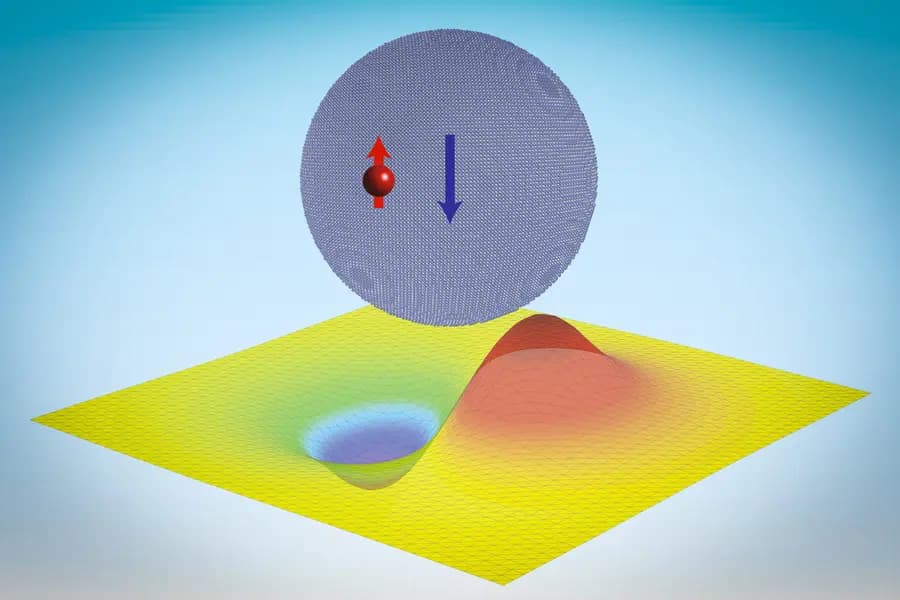
Quantum dots, tiny crystalline particles whose properties are determined by size and shape rather than composition, have become the focus of this study. While these particles usually bind electrons via electromagnetic forces, researchers have now theoretically demonstrated that they can also bind neutrons, even though the interaction happens over a much larger scale than the strong force’s usual range. The neutrons, in this case, form what the team has termed “neutronic molecules,” a state likened to Thomson’s plum pudding model of the atom, but on a much larger nanoscale.
This unexpected result, based on theoretical calculations and computational simulations, could have been identified earlier but was not due to the complexities involved. The computational challenge lies in the binding energy’s scale, which is minuscule compared to traditional binding. Using Green’s function, an analytical tool, the team showcased that neutrons could be captured by quantum dots larger than 13 nanometers in radius.
Further detailed simulations on materials like lithium hydride nanocrystals, which are considered for hydrogen storage, revealed the binding energy is influenced by the crystal’s dimensions, shape, and the nuclear spins of the nuclei and the neutron. This suggests that neutronic molecules might be tailored for specific purposes.
Creating neutronic molecules in a lab, however, poses significant challenges, including the need for extremely low temperatures. This task is left to other researchers with suitable equipment and expertise. These artificial molecules might allow for precision in controlling neutron states and could help in probing fundamental quantum mechanical issues.
Potential applications of this discovery are vast, from using neutronic molecules as a mediator in quantum computing systems to developing novel imaging techniques with neutron activation analysis. Unlike X-rays, neutrons interact more intensely with light elements, offering a complementary imaging method that can discern different isotopes within materials, a task beyond the scope of traditional chemical imaging.
This innovative work, supported by the U.S. Office of Naval Research, may lead to significant advancements in manipulating neutrons for quantum information systems and material analysis, exploring previously inaccessible aspects of the quantum realm.
The image is courtesy of researchers and ACS Nano.
The original story can be accessed here.
The research article published in ACS Nano can be accessed here.









Responses (0 )