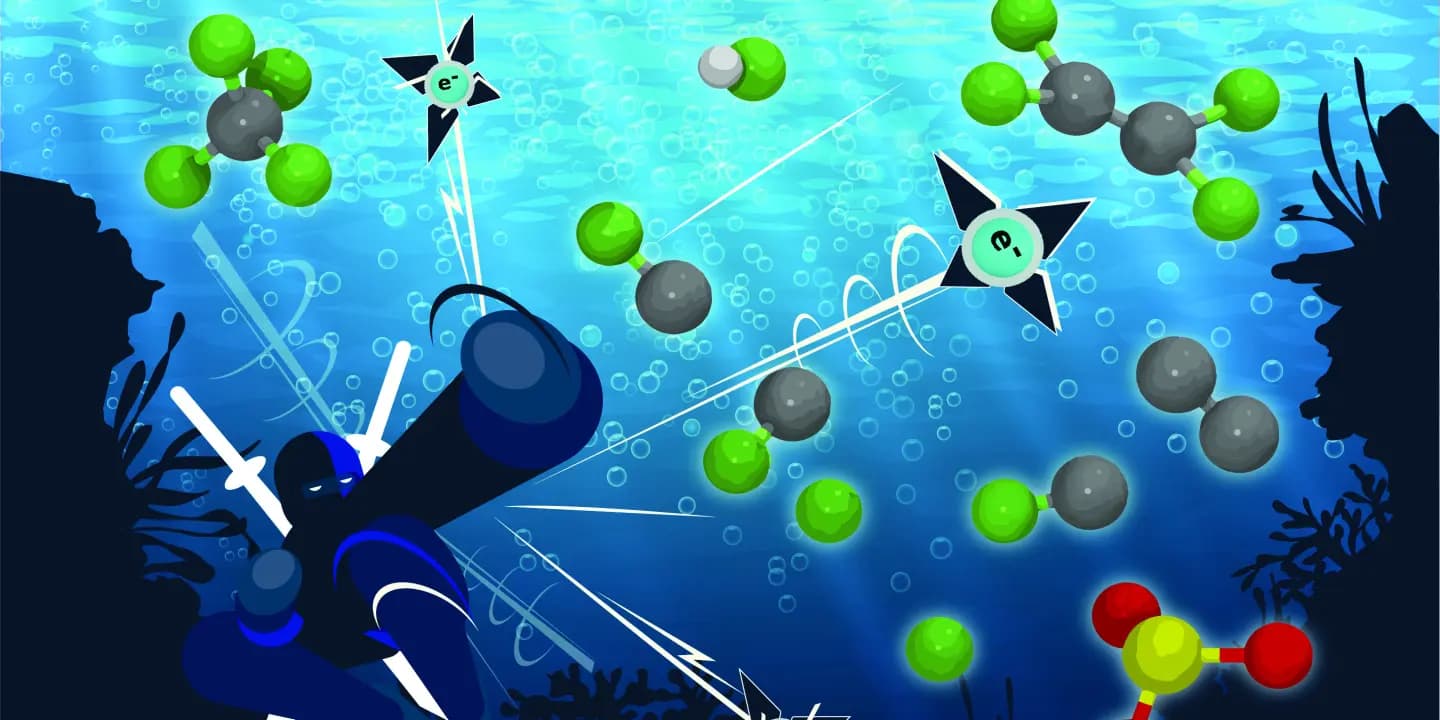
Excess electrons could help break the strong chemical bonds in products that contaminate water supplies
Synthetic chemicals known as per- and polyfluoroalkyls, or PFAS, contain bonds between carbon and fluorine atoms considered the strongest in organic chemistry. Unfortunately, the widespread use of these nonbiodegradable products since the 1940s has contaminated many water supplies across America.
Engineers at UC Riverside have now shown in modeling experiments that using excess electrons shatters the carbon-fluorine bond of PFAS in water, leaving by-products that might even accelerate the process. The paper is published in Physical Chemistry and Chemical Physics.
Impervious to heat, chemicals, and physical force, the carbon-fluorine bond makes PFAS ubiquitous in food packaging, stain, and water-repellent fabrics, polishes and waxes, firefighting foams, cleaning products, carpets, and thousands of other common household and industrial products. The Environmental Protection Agency estimates that most of the population has been exposed to PFAS that accumulate in the body over time because these “forever chemicals” do not biodegrade.
Sharma Yamijala, a postdoctoral researcher in the Marlan and Rosemary Bourns College of Engineering and first author of the paper, ran simulations on both perfluorooctanoic acid and perfluorooctanesulfonic acid molecules, the most common PFA contaminants in the environment, surrounded by water molecules. He found that they instantly lost their fluorine atom in the presence of excess electrons.
The PFA molecules broke down into an intermediate chemical species whose composition could further accelerate the decomposition of other PFA molecules. The reaction also formed a hydrogen fluoride molecule. Whether or not these short-chain molecules are carcinogens at typical concentrations in water has not yet been determined.
“In a real water treatment scenario, the excess electrons could come from metal-containing compounds placed in the water under ultraviolet radiation. The electrons from these compounds will interact with the PFA molecules and break them,” one of the authors said.
The simulations describe in precise detail a process that scientists have known is possible.
People knew you could do this but didn’t know how.
said Bryan Wong, an associate professor of chemical and environmental engineering and the paper’s senior author.
Our simulations define the bigger picture that we can refine to find ways to break down PFAs faster or more efficiently in the future.
The research was supported by grants from the U.S. Department of Energy and the National Science Foundation.
For the original paper, please visit the original research article.(https://doi.org/10.1039/C9CP06797C)









Responses (0 )